This part 1 of our comprehensive ‘Scientific Series’ covers all aspects of ‘Energy Storage’ which is required in the process of harnessing energy generated from Wind Power and Solar Power.
ENERGY STORAGE SYSTEM
Electricity is more versatile in use because it is a highly ordered form of energy
that can be converted efficiently into other forms. For example, it can be
converted into mechanical form with efficiency near 100 percent or into heat
with 100 percent efficiency. The heat energy, on the other hand, cannot be
converted into electricity with high efficiency, because it is a disordered form
of energy in atoms. For this reason, the overall thermal to electrical conversion
efficiency of a typical fossil thermal power plant is under 40 percent.
A disadvantage of electricity is that it cannot be easily stored on a large
scale. Almost all electrical energy used today is consumed as it is generated.
This poses no hardship in conventional power plants, where the fuel consumption
is varied with the load requirements. The photovoltaic and wind,
being intermittent sources of power, cannot meet the load demand all of the
time, 24 hours a day, 365 days of the year. The energy storage, therefore, is
a desired feature to incorporate with renewable power systems, particularly
in stand-alone plants. It can significantly improve the load availability, a key
requirement for any power system.
The present and future energy storage technology that may be considered
for stand-alone photovoltaic or wind power systems falls in the following
broad categories:
• electrochemical battery
• flywheel
• compressed air
• superconducting coil
Battery
The battery stores energy in the electrochemical form, and is the most widely
used device for energy storage in a variety of applications. The electrochemical
energy is a semi-ordered form of energy, which is in between the electrical
and thermal forms. It has one-way conversion efficiency of 85 to 90 percent.
There are two basic types of electrochemical batteries:
• the primary battery, which converts the chemical energy into the
electrical energy. The electrochemical reaction in the primary battery
is non-reversible, and the battery after discharge is discarded.
For this reason, it finds applications where high energy density for
one time use is needed.
• the secondary battery, which is also known as the rechargeable battery.
The electrochemical reaction in the secondary battery is reversible.
After a discharge, it can be recharged by injecting direct current
from an external source. This type of battery converts the chemical
energy into electrical energy in the discharge mode. In the charge
mode, it converts the electrical energy into chemical energy. In both
the charge and the discharge modes, a small fraction of energy is
converted into heat, which is dissipated to the surrounding medium.
The round trip conversion efficiency is between 70 and 80 percent.
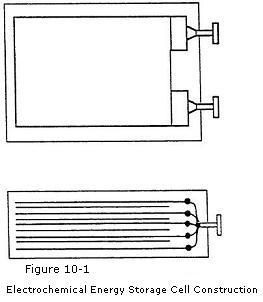
The internal construction of a typical electrochemical cell is shown in
Figure 10-1. It has positive and negative electrode plates with insulating separators
and a chemical electrolyte in-between. The two groups of electrode
plates are connected to two external terminals mounted on the casing.
The cell stores electrochemical energy at low electrical potentials, typically a few
volts. The cell capacity, denoted by C, is measured in Ampere-hours (Ah),
meaning it can deliver C amperes for one hour or C/n amperes for n hours.
The battery is made of numerous electrochemical cells connected in a
series-parallel combination to obtain the desired operating voltage and current.
The higher the battery voltage, the higher the number of cells required
in series. The battery rating is stated in terms of the average voltage during
discharge and the Ah capacity it can deliver before the voltage drops below
the specified limit. The product of the voltage and the Ah forms the Wh
energy rating it can deliver to a load from the fully-charged condition.
The battery charge and discharge rates are stated in unit of its capacity in Ah.
For example, charging a 100 Ah battery at C/10 rate means charging at 10
A rate. Discharging that battery at C/2 rate means draining 50 A, at which
rate the battery will be fully discharged in 2 hours. The State of Charge (SOC)
of the battery at any time is defined as the following:
Types of Batteries
There are at least six major rechargeable electro-chemistries available today.
They are as follows:
• lead-acid (Pb-acid).
• nickel-cadmium (NiCd).
• nickel-metal hydride (NiMH).
• lithium-ion (Li-ion).
• lithium-polymer (Li-poly).
• zinc-air.
New electro-chemistries are being developed by the United States Advance
Battery Consortium for a variety of applications, such as electric vehicles,
spacecraft, utility load leveling and, of course, for renewable power systems.
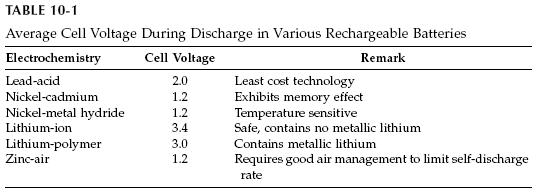
The average voltage during discharge depends on the electrochemistry, as
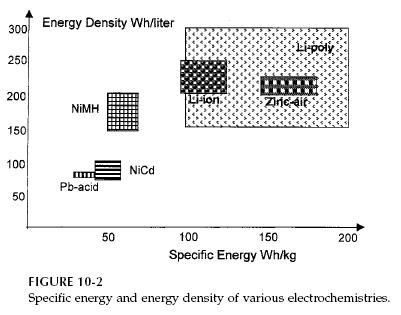
listed in Table 10-1. The energy density of various batteries, as measured by
Wh capacity per unit mass and per unit volume, are compared in Figure 10-2.
The selection of the electrochemistry for a given application is a matter of
performance and cost optimization.
Some construction and operating features of the above electro-chemistries
are presented in the proceeding parts 2 and 3 of this ‘Scientific Series’.

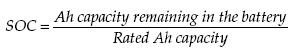



Nice start to your series. I hope you plan on including flow batteries! Unlimited charge-recharge cycles, long life – to 20 years, no disposal or recycling issues, currently installed at wind energy locations, etc..
That is very informative. Thank you for sharing that.
Nice summary